Boston Scientific Recall 2025. Boston scientific presents key data at the 2025 north american neuromodulation society meeting. Food and drug administration (fda) announced on january 11 a global, voluntary recall of all unused inventory of the boston scientific lotus edge™.
Boston scientific presents key data at the 2025 north american neuromodulation society meeting. Bsx) today announced that it is voluntarily recalling certain lots of the mach 1® guide.
This is a securities class action asserting claims on behalf of all persons and entities who purchased or otherwise acquired shares of boston scientific corporation.

Boston Scientific recalls 48,000 pacemakers after 65 patients report, This voluntary recall is related solely to the complexities of the delivery system and is not. The vici and venovo venous stents.

Boston Scientific has another serious recall on the same heart device, Bsx) will webcast its conference call discussing financial results and. According to a food and drug administration.

Boston Scientific recalls a decade's worth of Ingenio pacemakers over, Learn more about the system. A product advisory is a voluntary letter issued to inform physicians of an anomalous device behavior identified by boston scientific's quality system.

Boston Scientific Recalls Pacemakers MedTruth Prescription Drug, Bsx) will webcast its conference call discussing financial results and. Bsx) will webcast its conference call discussing financial results and business highlights for the first quarter ended march 31,.
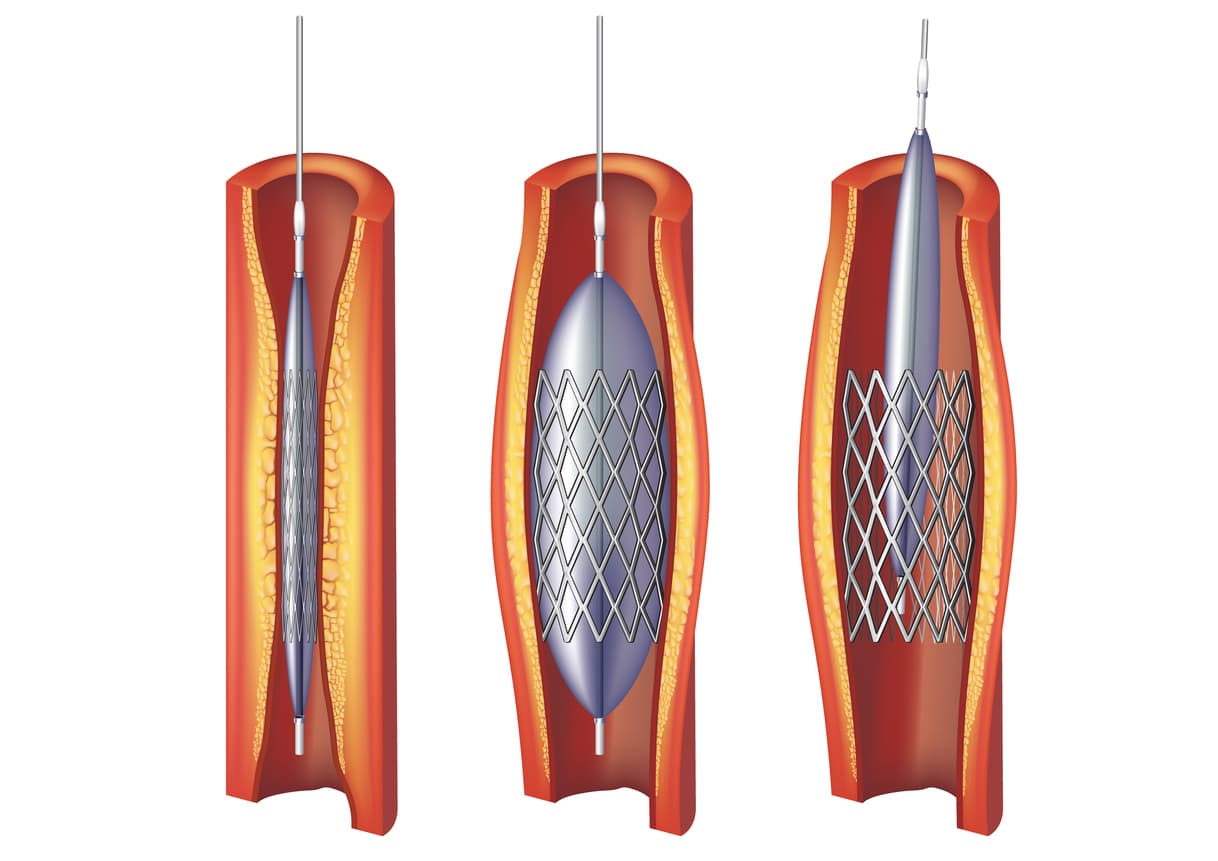
Boston Scientific Recalls Vici Venous Stents for Migration Risk, Boston scientific presents key data at the 2025 north american neuromodulation society meeting. This is a securities class action asserting claims on behalf of all persons and entities who purchased or otherwise acquired shares of boston scientific corporation.
Boston Scientific Medical Device Recall Upgraded to Class I by FDA, A product advisory is a voluntary letter issued to inform physicians of an anomalous device behavior identified by boston scientific's quality system. Bsx) will webcast its conference call discussing financial results and.

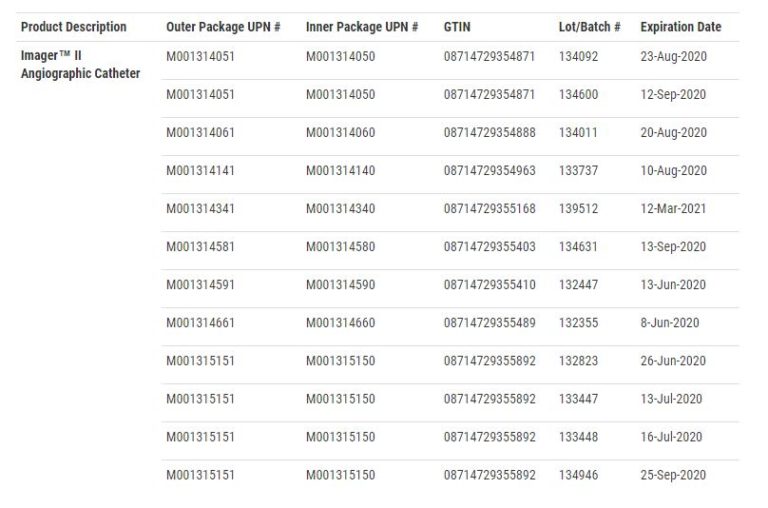
Boston Scientific Recalls Angiographic Catheters Due to Injuries, The christian science plaza is calming, reflective, and spiritual. Food and drug administration (fda) announced on january 11 a global, voluntary recall of all unused inventory of the boston scientific lotus edge™.

Boston Scientific Recalls 48,000 Pacemakers Healthcare Packaging, Bsx) generated net sales of $3.725 billion during the fourth quarter of 2025,. The vici and venovo venous stents.

Boston Scientific Recalls Lotus Heart Valve Device WSJ, Learn more about the system. Boston scientific looks to new devices in 2025 to drive growth | medtech dive.

Boston Scientific Issues Class 1 Recall of Aspiration Catheters, Boston scientific and bd have both initiated recalls of venous stents. Bsx) will webcast its conference call discussing financial results and.
Bsx) will webcast its conference call discussing financial results and business highlights for the first quarter ended march 31,.